
Infect Control Hosp Epidemiol 2001 22:433–6. Effectiveness of surveillance of central catheter-related bloodstream infection in an ICU in Korea. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. A cohort and case-control study in critically ill patients.
Outcomes of primary and catheter-related bacteremia. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Blot SI, Depuydt P, Annemans L, et al.Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ.Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA.Prevention of intravascular catheter-related infections. Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX. (Arrow International’s parent company) for a refund.Ĭustomers with questions should call Teleflex customer service at 86 or email Additional Resources:
 Return affected products to Teleflex Inc. Stop using and distributing affected products. The letter recommended the following actions: On October 27, 2022, Teleflex and Arrow International, LLC sent customers an Urgent Medical Device Recall letter.
Return affected products to Teleflex Inc. Stop using and distributing affected products. The letter recommended the following actions: On October 27, 2022, Teleflex and Arrow International, LLC sent customers an Urgent Medical Device Recall letter. Triple lumen central line plus#
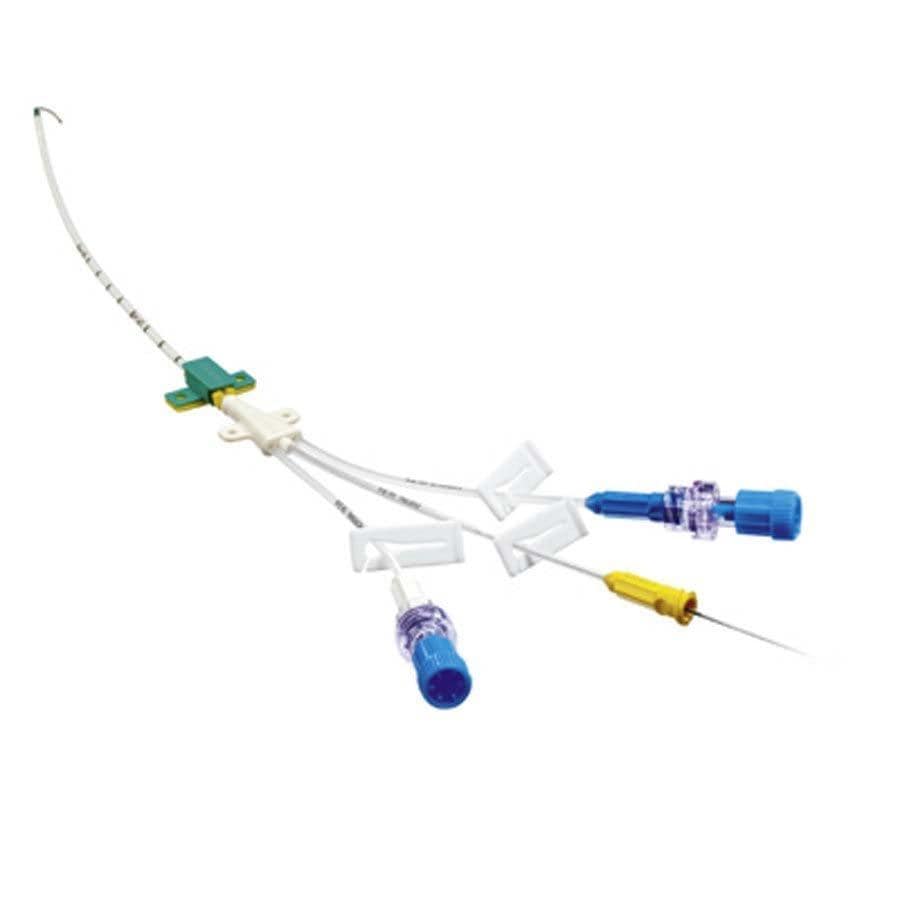
Distributors of the Arrow MAC Two-Lumen Central Venous Access Kits Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits. Health care personnel providing care using the Arrow MAC Two-Lumen Central Venous Access Kits Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits. People who receive care using the Arrow MAC Two-Lumen Central Venous Access Kits Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits. Teleflex/Arrow International LLC has reported no injuries or deaths related to this issue. Use of affected devices may cause bleeding, fluid leakage, delayed treatment, infection, air in the blood vessels (air embolism), other serious injuries or death. Teleflex and their subsidiary Arrow International, LLC are recalling the Arrow MAC Two-Lumen Central Venous Access Kits Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits for the risk of a cross-lumen leak caused by inadequate connections between the top and bottom housings of the Micro Clave Clear Connectors included in the kits. It is not intended to be used as a treatment for existing infections. The Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen CVC Kit is intended to provide short-term (less than 30 days) protection against catheter-related bloodstream infections. The Arrow MAC Two-Lumen Central Venous Access Kit is intended to permit short term (less than 30 days) venous access and catheter introduction to the central circulation. Date Initiated by Firm: October 27, 2022. Product Models: See Recall Database entry. Product Name: Arrow MAC Two-Lumen Central Venous Access Kits Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter (CVC) Kits. Use of these devices may cause serious injuries or death. The FDA has identified this as a Class I recall, the most serious type of recall.







 0 kommentar(er)
0 kommentar(er)
